Medical devices sold in the US are regulated by the FDA (Food and Drug Administration), and in the EU, they are regulated by the European Commission and the competent authorities in each of the member states. Medical devices must meet applicable regulations for the US or EU, depending on which market the device will be made available on.
For you as a patient or consumer, it means that any medical device you use must have the proper certification before it is made available.
Medical device classification according to risk
Different countries in the world have different regulatory requirements, and these depend on the risk the product in question exposes the patients or users to. However, one principle is very commonly used; the higher the risk, the higher the classification of that medical device. This also means that the manufacturers need to
All medical devices are categorised according to classification systems. In the European Union, there are four major classes according to the Medical Device Regulation (MDR):
- Class I
- Class IIa
- Class IIbÂ
- Class III
In the US, the principles of medical device classification are similar but not the same. The US have class 1, 2, and 3 products. Even if the systems are similar, the rules are not identical.
The differences between the EU and the US may seem small, but sometimes, products end up in different classes in these two markets. Medical devices have to be classified individually by the manufacturer for the markets they will be sold to. Performing the classification for the US market is done following the database on the FDA website.
How to perform medical device classifications
The people working on the classification of medical devices will use annex VIII of the Medical Device Regulation. They are required to have technical documentation with a classification rationale in order to launch a product to the EU market.
Another important point is that regardless of classification, medical devices will have to meet the General Safety and Performance Requirements, as well as harmonised standards.
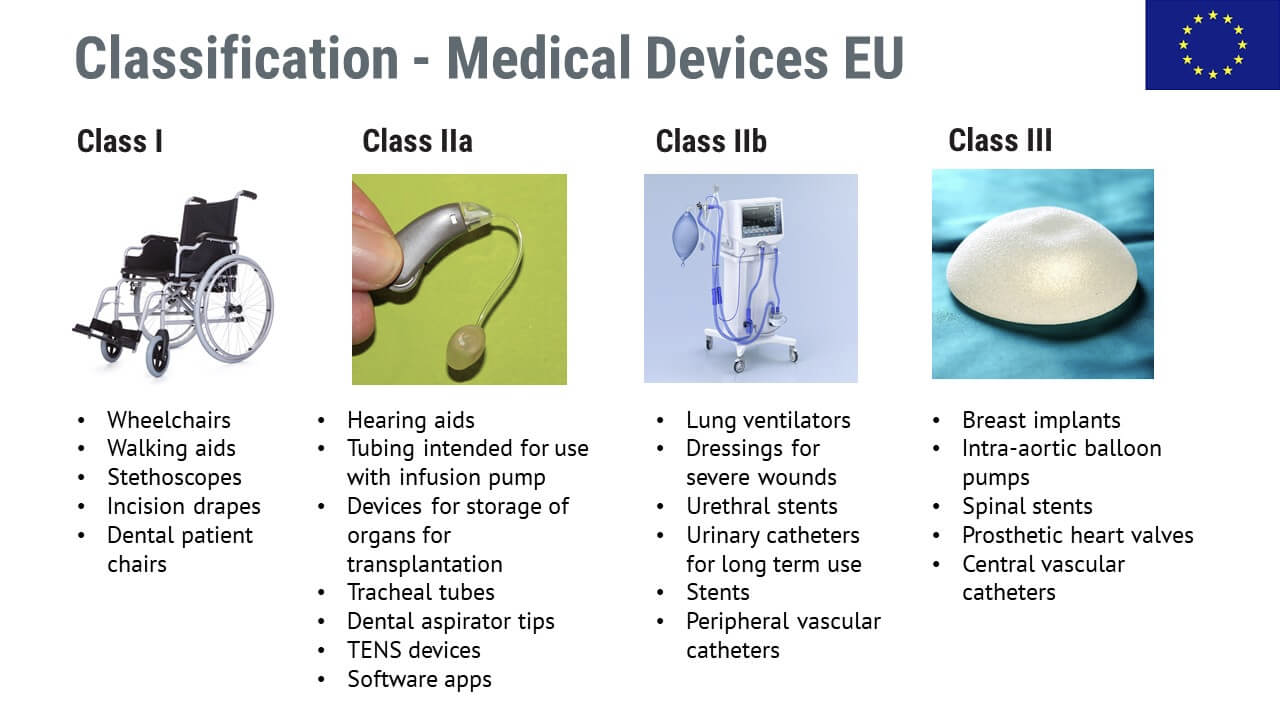
Products sorted by medical device classification
Here are some examples of medical devices divided by classes. Essentially, lower-class devices such as wheelchairs and walking aids involve lower risks, while class IIb and class III devices such as lung ventilators or spinal stents have higher risks.
Risk management for class I devices is often less rigorous, focusing on ensuring basic safety and performance standards. For class II devices, risk management may involve more stringent testing and quality control measures.
Class III and IV devices, due to their high-risk nature, require a more comprehensive risk management approach, including clinical trials and post-market surveillance.
How do I find out which classification a medical device has?
As a user, you will not always be able to find out which classification a medical device has. But you could look for the declaration of conformity for products that are sold on the EU market. It will sometimes contain the classification. You can, however, check if a medical device meets the applicable regulations to be placed on the market.
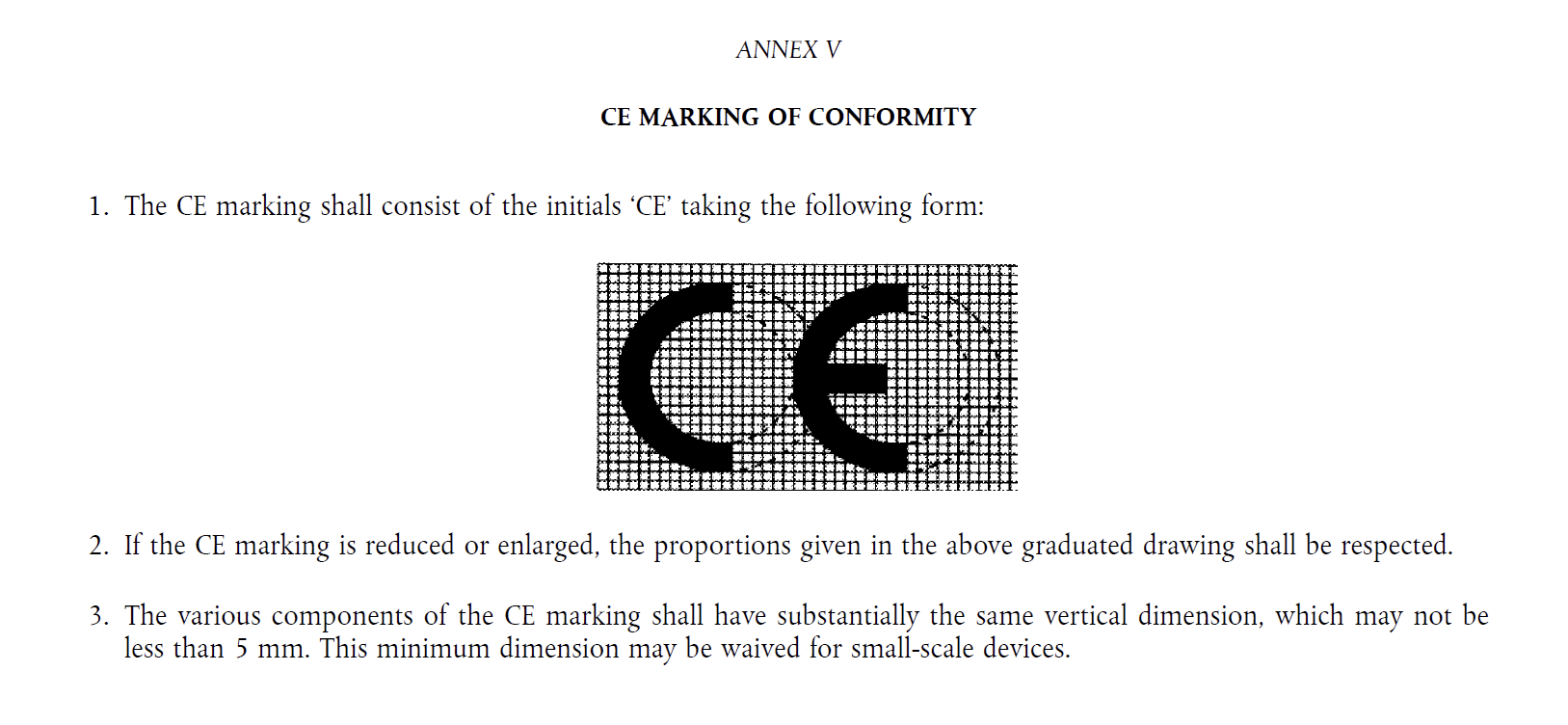
If your medical device has a CE marking, this shows that the product has fulfilled applicable regulations in the EU. Please note that the CE marking should look like it does in the Medical Device Regulation, Annex V. If the proportions or dimensions of the CE-mark are not in line with the MDR, then the product may have been unlawfully placed on the market.
How can users mitigate the risks associated with different classes of medical devices?
As a user, you, yourself can do several things to mitigate risks associated with various medical devices that you use.
Reading through and following the manufacturer's instructions for use is the first thing you can do. If needed, you should ensure that you receive adequate training on how to use the device correctly. This is especially true with more complex medical devices since misuse can lead to harm.
Regular maintenance and inspection of the device, as well as reporting any malfunctions to the manufacturer, can also help reduce risks for you and other future users.
And as always, it's also important to only use the device for its intended purpose and to regularly check for any software updates (if applicable).
